Alzheimer’s disease is a major public health concern due to the impact of the disease on patients and their families, but also because of a lack of efficient treatments. The disease can have two forms: a rare genetic one, and one whose occurence is linked to aging.
No matter the type, Alzheimer’s disease is characterized by several issues:
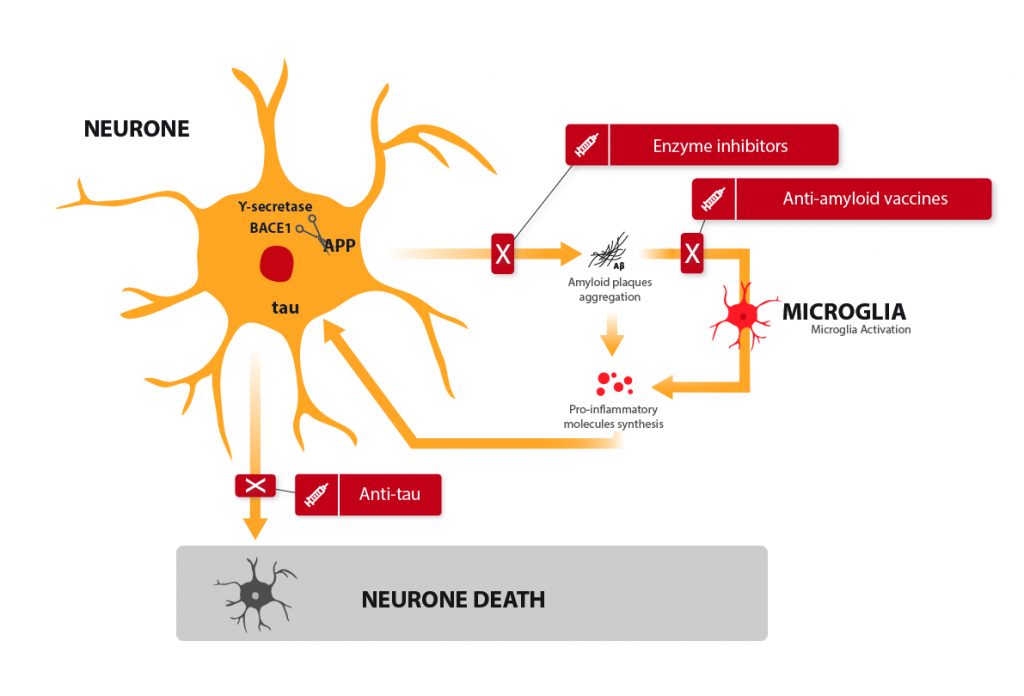
- Agregation of a small protein, the beta-amyloid, also called A-beta, forming plaques between the neurons: the “amyloid plaques”. They prevent proper communication between the neurons.
- Accumulation of the tau protein, a key protein for carrying molecules into the neurons and maintaining their structural integrity. Enzymes abnormally modify the tau protein by adding too many small groups of phosphate.
- Inflammation, in response to both of these issues. It grows and grows as the disease progresses and plays a role in the death of neurons.
Here is the current theory as to how this happens, the amyloid cascade theory: A-beta, when accumulating to form plaques, triggers several detrimental signals which bring on a modification of the tau protein, inflammation, and eventually, neuronal death. This is when the first symptoms of the disease start appearing. It starts with memory loss, disorientation, losing the sense of time… That is when most patients become concerned, which is an issue given the lack of curative treatments.
To remedy the issue, many ideas have led to potentials solutions being currently developped.
Anti-amyloid “vaccines”
It might be a bit bold to speak of a vaccine, but the term was jointly adopted by an equally enthusiastic public and scientific community: it consists of an injection of antibodies to the patient. The antibodies recognize A-beta and allow its elimination. The mechanism is very similar to what we know of vaccines. The process is actually more complex, it can be active (1, 2) (with our cells acting on their own to get rid of the amyloid) or passive (3) (the drug itself prevents the formation of amyloid plaque.) This promising and innovative therapeutic lead is being developped.
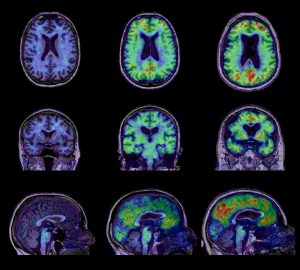
Metabolic and anatomical differences between, from right to left, a control group, patients with mild dementia, and Alzheimer’s patients.
However, the first clinical trials do not seem as promising as it was hoped(4, 5, 6, 7). Of course, it somehow lowers the amount of amyloid plaque, but not enough to significantly reduce the symptoms. Many strategies are being developed in order to maximize the efficiency of these vaccines while lowering their side effects.
Enzyme inhibitors
A-beta is a small peptide from a bigger protein called APP. In order to make A-beta, APP must be cut up by two enzymes: gamma-secretase and beta-secretase (also called BACE-1). Still with the aim of reducing amyloid plaque, treatments have been developed to reduce the activity of those enzymes, to prevent A-beta formation from the very begining (8, 9). Gamma-secretase inhibitors were a colossal failure, with significant side-effects and a degradation of the patients’ state. BACE-1 inhibitors are still in the clinical testing phase, and conclusions are expected by the end of 2018.
Anti-tau vaccines
An approach similar to that of the anti A-beta vaccines was developed, targeting tau, the second protein to blame for neuronal death. Many of these approaches were dismissed, as they showed either important side effects or no significant efficiency. One clinical trial is still ongoing (10), the results are awaited during the year 2019.
Another approach is being considered, which is based on the anti-platelet properties of a drug used against malaria. This aims to prevent tau accumulation in the neurons (11). So far, the outcomes are promising, although some are looking into reducing the side effects of the drug.
Anti-inflammatory treatments
Inflammation is the real issue behind Alzheimer’s disease: when in distress, neurons signal cells which have no other alternative but to trigger an inflammation. This is normally a temporary and beneficial reaction for the body. In the brain of an Alzheimer’s patient, however, the process starts with the distress of neurons, is amplified by the microglia, spreads to the closest neurons which signal in turn the microglia again, creating an “inflammasome”: a large, self-sustained inflammation zone leading to neuronal death.
This is why anti-inflammatory treatments are an interesting therapeutic lead. Unfortunately, most of the research in this had to be abandoned due to lack of efficiency.
More surprising therapies: ultrasounds
Of course, anti-amyloid, anti-tau and anti-inflammatory treatments are good therapy candidates. They are relevant to the commonly accepted hypothesis of the amyloid cascade among the scientific community (A-beta is harmful, modifies tau, kills neurons and encourages inflammation).
However, what we still don’t know is why the A-beta starts forming plaques or why our brains reach a stage where they decide to start erasing memories when there is too much plaque and too many neurons gone. Research is currently looking into alternative therapies such as ultrasounds.
When A-beta has formed plaques, it becomes very difficult to get rid of them, despite all the efforts of the microglia. Ultrasounds allow a temporary opening of the blood-brain barrier: they trigger vibrations in the cells that block bigger molecules from accessing the brain, and forces them to retract for a few seconds. This helps amyloid plaques cross the blood-brain barrier to be eliminated later on (12). With just a few ultrasound sessions, the plaques are almost completely gone, with drastic cognitive ameliorations! Animal testing is still ongoing but is proving to be very promising so far. This would also offer a non-medicinal treatment.
Listing all the therapies currently being investigated would take longer, but fear not, research is getting closer to a cure! If you wish to know more about the treatments currently being developed, feel free to visit AlzForum, a website on Alzheimer’s disease.
- Pride M, Seubert P, Grundman M, Hagen M, Eldridge J, Black RS. Progress in the active immunotherapeutic approach to Alzheimer’s disease: clinical investigations into AN1792-associated meningoencephalitis. Neurodegener Dis. 2008;5:194–196.
- Hock C, Konietzko U, Paspassotiropoulos A, Wollmer A, Streffer J, von Rotz RC, Davey G, Moritz E, Nitsch RM. Generation of antibodies specific for β-amyloid by vaccination of patients with Alzheimer disease. Nat Med. 2002;8:1270–1276.
- Abdelrahman Ibrahim Abushouk, Ahmed Elmaraezy, Amro Aglan, Reham Salama, Samar Fouda, Rana Fouda, and Ammar M. AlSafadi Bapineuzumab for mild to moderate Alzheimer’s disease: a meta-analysis of randomized controlled trials. BMC Neurol. 2017; 17: 66.
- Wisniewski T. Practice point commentary on “Clinical effects of Aβ immunization (AN1792) in patients with AD in an interrupted trial. Nat Clin Prac Neurol. 2005;1:84–85
- Ferrer I, Boada RM, Sanchez Guerra ML, Rey MJ, Costa-Jussa F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathol. 2004;14:11–20
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2005;9:448–452.
- Michael Gold Phase II clinical trials of anti–amyloid β antibodies: When is enough, enough? Alzheimer’s Dement (N Y). 2017 Sep; 3(3): 402–409
- Kennedy ME, Stamford AW, Chen X, Cox K, Cumming JN, Dockendorf MF, Egan M, Ereshefsky L, Hodgson RA, Hyde LA, Jhee S, Kleijn HJ, Kuvelkar R, Li W, Mattson BA, Mei H, Palcza J, Scott JD, Tanen M, Troyer MD, Tseng JL, Stone JA, Parker EM, Forman MS. The BACE1 inhibitor verubecestat (MK-8931) reduces CNS β-amyloid in animal models and in Alzheimer’s disease patients. Sci Transl Med. 2016 Nov 2;8(363):363ra150
- Coric V, van Dyck CH, Salloway S, Andreasen N, Brody M, Richter RW, Soininen H, Thein S, Shiovitz T, Pilcher G, Colby S, Rollin L, Dockens R, Pachai C, Portelius E, Andreasson U, Blennow K, Soares H, Albright C, Feldman HH, Berman RM. Safety and Tolerability of the γ-Secretase Inhibitor Avagacestat in a Phase 2 Study of Mild to Moderate Alzheimer Disease. Arch Neurol. 2012 Aug 13;:1-12.
- Kontsekova E, Zilka N, Kovacech B, Novak P, Novak M. First-in-man tau vaccine targeting structural determinants essential for pathological tau-tau interaction reduces tau oligomerisation and neurofibrillary degeneration in an Alzheimer’s disease model. Alzheimers Res Ther. 2014;6(4):44.
- Wischik CM, Staff RT, Wischik DJ, Bentham P, Murray AD, Storey JM, Kook KA, Harrington CR. Tau aggregation inhibitor therapy: an exploratory phase 2 study in mild or moderate Alzheimer’s disease. J Alzheimers Dis. 2015;44(2):705-20.
- Leinenga G, Götz J. Scanning ultrasound removes amyloid-β and restores memory in an Alzheimer’s disease mouse model. Sci Transl Med. 2015 Mar 11;7(278).


![[Video] Eurosymposium on Healthy Ageing, Brussels, 2018 Eurosymposium on Healthy Aging](http://www.longlonglife.org/wp-content/uploads/2019/07/P1310252-218x150.jpg)