Senescence and chromosomal abnormalities: a new perspective for longevity
Cell senescence, the state of cell cycle arrest, is one of the most studied causes of aging. Targeting senescence has become, in just a few years, a major strategy in delaying aging. The latest study, published this month in Nature Communication, has found a correlation between cell senescence and aneuploidy. Aneuploidy is an abnormal number of chromosomes in a cell, which is caused by missegregation of the chromosomes, prior to the division of the mother cell into two daughter cells. As quoted by the researchers in their paper, the purpose of the study was to understand the relationship linking this chromosomal abnormality and senescence, to develop a new approach for targeting senescence and aging.
A new study to target senescence
The experiments conducted on human fibroblasts from patients’ skin revealed that as they age, the cells divide more slowly and the number of defects following these divisions was increasing; aneuploidy is one of them. Observations of fibroblasts from different generations have, in fact, revealed that this phenomenon increases with age, due to the dysfunction of the components that regulate the cell cycle. Scientists have in particularily focused on the transcription factor FoxM1 (Forkhead box M1), which regulates the transition from the cell preparation phase to mitosis itself. This is an important control point that checks if the cell has replicated its DNA and is ready to divide: when FoxM1 is repressed, this control does not work well and is associated with mitosis defects. Consistent with what was previously shown, the researchers also proved that when FoxM1 was activated, cells with an elderly phenotype or with progeria (a severe premature aging syndrome) were rescued and properly divided. But how does this relate to aneuploidy? The experiments of the team showed that this chromosomal abnormality was associated with cell cycle arrest, and therefore probably with a FoxM1 regulation problem leading to total fibroblast senescence.
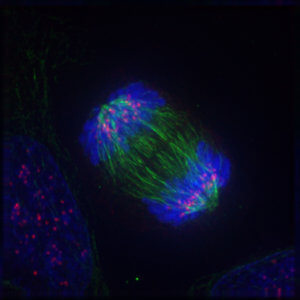
Inadequate chromosome migration and senescence
The aberrant segregation of chromosomes from elderly cells is therefore associated with the suppression of FoxM1. Previous studies have assumed that senescent cells exit the cell cycle, do not die, but cannot divide either. The study showed that missegregation of chromosomes was sufficient to trigger complete and permanent cell cycle arrest and thus induce senescence. In summary, poor chromosome segregation before mitosis correlates with FoxM1 suppression and early onset of senescence.
The normal loss of FoxM1 is due to cellular stresses, such as genomic instability or loss of proteostasis, both major causes of aging. By restoring FoxM1, researchers were able to delay the senescence of elderly fibroblasts or those with progeria syndrome. This implies that the proper regulation of FoxM1 could become an interesting therapeutic avenue to fight against cell senescence and bring many benefits over the lifetime in good health.
Reference:
[1] Joana Catarina Macedo, Sara Vaz, Bjorn Bakker, Rui Ribeiro, Petra Lammigje Bakker, Jose Miguel Escandell, Miguel Godinho Ferreira, René Medema, Floris Foijer & Elsa Logarinho, FoxM1 repression during human aging leads to mitotic decline and aneuploidy-driven full senescence, NATURE COMMUNICATIONS | (2018) 9:2834 | DOI: 10.1038/s41467-018-05258-6
Anne Fischer

Author
Auteur
Anne is studying medicine science at the Institute of Pharmaceutical and Biological Science in Lyon and she has graduated with a Bachelor’s degree in molecular and cellular biology at the University of Strasbourg.
More about the Long Long Life team
Anne étudie les sciences du médicament à l’Institut des Sciences Pharmaceutiques et Biologiques de Lyon. Elle est titulaire d’une licence en biologie moléculaire et cellulaire de l’Université de Strasbourg.
En savoir plus sur l’équipe de Long Long Life