Pillarcell microfluidic system for stem cell differentiation
Developing universal tools for stem cell control
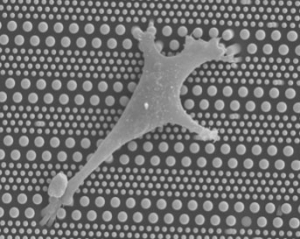
Stem cell differentiation control will be a key technology for future medical treatment and in the fight against human aging, since stem cells have the inherent particularity to self-renew along a life cycle. The Pillarcell research project aims to demonstrate the advantages of using microfluidic technologies to finely control stem cell migration and differentiation.
“Nature does nothing uselessly” (Aristotle: I.1253a8). This is also true for the human body, an organism which is vastly complex and made up of highly-synchronized sub-systems and a huge number of individual cells. When body tissue or cells are placed in a dish, a flask or a multi-well plate, they undergo substantial changes of the cellular micro-environment. Their extracellular matrix becomes much simpler, soluble factors and nutrients usually present in their environment are provided differently and cell-to-cell contact is modified. Such changes have important consequences on the performance of cell-based assays, tissue engineering and regenerative medicine. Although a large amount of research has been done to improve in-vitro culture conditions, there is still no satisfactory technology that correctly mimics real in-vivo cellular micro-environments.
One reason is the lack of general platforms that allow for the high-precision regulation of cellular micro-environments. Micro-engineering techniques are now widely used to fabricate surface textured culture substrates and synthetic extracellular matrix on the one hand, and microfluidic devices for the dynamic control of soluble factors on the other hand. In this project, we are undertaking a systematic investigation of cell migration and differentiation on patterned micro and nano-pillar arrays, with or without integration into microfluidic devices. By changing the geometrical parameters of the pillars, we particularly focus on the role of substrate stiffness on cell migration and differentiation.
We are working on this research project as part of a multidisciplinary consortium comprised of three academic research teams (one from the ENS specialized in nanofabrication technologies for cell biology, one from the Curie Institute specialized in cell polarity and migration on patterned surfaces, and another from the University of Paris-Diderot (Institut Jacques Monod) specialized in the biophysics of cell-based assays).
Our system can also be useful for other types of cell-based assays, including adhesion, proliferation, differentiation, and apoptosis. Since the fabrication technology we propose can be used for large scale manufacturing, it will be easy to convert our prototypes into industrial products. To this end, we anticipate a number of clinical applications of such products, including tissue engineering and wound healing.
Hadrien Vielle

Author
Auteur
Hadrien is an engineer and was trained in biology, physics and bio-engineering at the École Polytechnique féminine in Paris.
More about the Long Long Life team
Hadrien est aujourd’hui ingénieur polyvalent après une formation en biologie, physique et bio-ingénierie à l’École Polytechnique féminine.
En savoir plus sur l’équipe de Long Long Life