Metabolism: a fundamental but fragile mechanism

To understand metabolic diseases, it is necessary to know what is defined by metabolism. Metabolism, in the broadest sense, includes all the chemical reactions that take place in our bodies. It is divided into two parts: anabolism and catabolism.
Anabolism corresponds to the chemical reactions of our body leading to the production of new functional molecules. This includes the synthesis of new proteins or DNA replication. On the other hand, catabolism corresponds to degradation reactions. For example, sugars and fats are degraded to use as energy. The recycling of damaged proteins also falls into this category.
In addition, for our body to function properly, it is important that these two phenomena are in balance. Here is a simple example to illustrate it. Let’s take a cell that synthesizes its proteins faster than it can recycle them. One could then imagine that at first, everything would go well. Unfortunately, after a while, the proteins get damaged. They therefore become less efficient or even dangerous for the cell. In addition, the cell recycles them too slowly. It cannot therefore recover amino acids, the building blocks of proteins. It therefore becomes unable to synthesize new functional proteins: old proteins accumulate and the cell dies.
The diseases that we will describe in this article all have as their cause a disruption of this production/degradation balance following aging. Together we will see three of the most recurrent examples, which can impact any part of our bodies.
Metabolism and aging: diabetes happens when your metabolism is out of fuel
The first age-related metabolic disease we will address is diabetes. There are three types of them. A distinction is made between type I diabetes, type II diabetes and gestational diabetes. The diabetes that is reported as we age is usually type II diabetes[1], but we will get back to that.
Metabolism and aging: insulin, a metabolic switch
First of all, what is diabetes? To understand it, we must first look at the body’s mechanism for delivering glucose, the main fuel of cells, to all our organs. When we eat, our blood glucose levels increase. However, for a cell to absorb it, it must receive a hormonal signal: insulin. This hormone is secreted by very specific cells of our pancreas, the β cells. These cells are able to feel the blood glucose level very accurately. When it rises, they secrete insulin and our cells can absorb the sugar until its level drops. Insulin also stimulates most of the anabolic activities, mentioned in the introduction, of our body, thus indicating to our cells that the necessary energy is available[2]. Thus, insulin acts as an organizer of a large part of our metabolism. When insulin no longer functions, our bodies fail: that is when diabetes happens.

Metabolism and aging: insulin function is altered with age
Aging is one of the main factors that will degrade this system, which is essential for our body’s energy management[3]. For a signal to no longer perform its function, there may be two reasons: it is not sent or not received. The same applies to the insulin signal. The two main causes of diabetes are related to a problem with sending or receiving the hormone. It is therefore a question of the sending of insulin by our cells β or the ability of our organs to interpret the signal[4].

Metabolism and aging: β cells out of their depth
First of all, let’s deal with communication problems, affecting the β cells. In type I diabetes, they are completely destroyed by the immune system[5]. This is the so-called “hereditary” form of diabetes because it most often occurs in young people and lasts a lifetime. This phenomenon can also be observed during aging. In the case of type II diabetes in the elderly, the number of β cells is often impacted, but to a lesser extent (30 to 40% less than in healthy individuals). The reasons identified are also different, it would be a predisposition of these cells to die quickly[6] or an inability to practice autophagy[7]. This phenomenon is also accompanied by an inability of patients’ β cells to secrete enough insulin when glucose levels rise[8]. Thus, being less numerous and less efficient, the patients’ β cells can no longer perform their function and insulin signals are no longer sufficient.
Metabolism and aging: when organs become less sensitive
The second phenomenon that makes insulin signals defective is when our bodies stop receiving them. This may be related to the dysfunction of insulin receptors, small proteins that allow insulin to bind to the surface of cells[9]. Sometimes, this receiver is even systematically destroyed[10].
There may be a second reason for the poor reception of insulin signals by our bodies, and it is much less direct than the simple dysfunction of the receptor. The insulin signalling pathway is a complex phenomenon within the cell itself, involving many proteins[11]. However, these proteins may already be present elsewhere, or absent. Thus, many phenomena often associated with age, such as mitochondrial dysfunction[12], inflammation[13] or response to protein misfolding[14], use the proteins that insulin needs to get its message across, making them inaccessible. By combining these two causes, the cells of diabetic patients become insensitive to insulin, which can no longer fulfill its role.

To sum it up, aging can cause the apparition of diabetes by preventing our bodies from properly absorbing glucose. The phenomenon is due to a communication dysfunction between the pancreas and the body through insulin.
Metabolism and aging: diabetes, consequences and treatment
As we can imagine, the consequences of an inability to get glucose properly into our cells are important for metabolism, and therefore for the health of patients. Diabetes causes, for example, severe fatigue, weight loss despite adequate nutrition, vision problems, vulnerability to infections[15]… Fortunately, there are many treatments available. The most common is the injection of insulin, which replaces missing or less efficient β cells. This also allows the cells to obtain a sufficiently high insulin level despite their inability to feel it properly[16]. In addition, a healthier lifestyle or the taking of certain molecules when diabetes develops prevents it from progressing[4]. Finally, there are many promising approaches, either to restore the number of cells β[17] or to make the body more sensitive to insulin[18]. This could make it possible, in the future, to eradicate this disease disrupting our metabolism.
Metabolism and aging: gout happens when metabolic waste accumulates

As defined above, metabolism is a set of production and degradation processes. Unfortunately, not everything it produces is infinitely recyclable. Like human production systems, metabolism creates waste. The comparison does not stop there. Indeed, if it accumulates, this waste can become highly toxic. As we will see, gout results from poor metabolic waste management. Its accumulation near the joints of our body will then poison the cells in them, just as an open landfill would poison the surrounding environment.
Metabolism and aging: uric acid is your metabolism’s toxic waste
DNA, the carrier of our genetic information, is essential to our body. Nevertheless, it is sometimes necessary to degrade it. Things are getting more complicated here. Indeed, some of its constituents, the purines to be more exact, are not totally degradable. In humans and large primates, their degradation results in the formation of a potentially toxic molecule: uric acid[19].
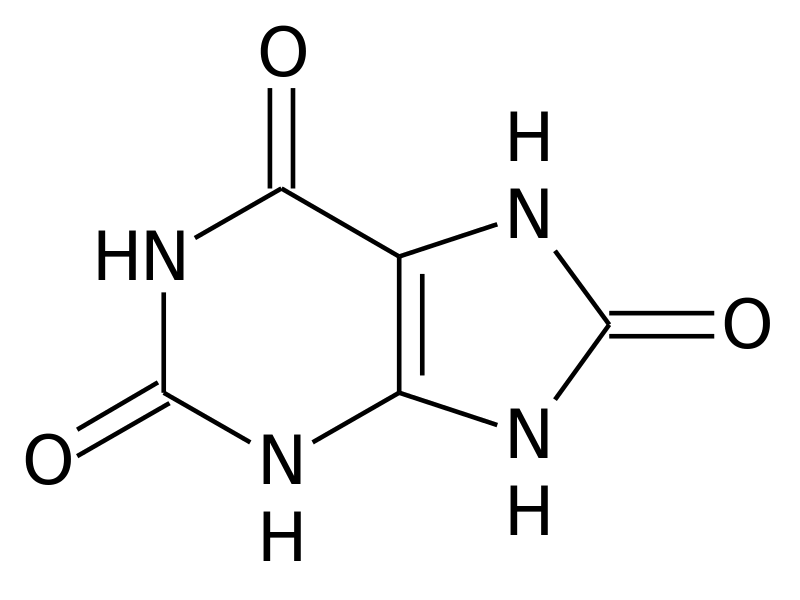
But how and why does it accumulate? For waste to accumulate, there are two ways: either too much is generated or it is not disposed of quickly enough. In the case of patients with gout, the accumulation is in most cases due to a problem with uric acid excretion[20]. Normally, our bodies get rid of uric acid through the kidneys (i.e. via the urine) or through the intestines[21]. Unfortunately, as we age, these processes can be deregulated[22]. This leads to an accumulation of uric acid in the blood, which then becomes capable of forming crystals[23]. It is these crystals that are toxic to our cells and will cause the development of gout.

Metabolism and aging: gout, a poison for our joints
As they accumulate in the joints, the crystals cause impressive inflammation. But why? Our cells are able, in order to study their environment, to absorb part of what surrounds them to examine it. In the case of gout, this is what will cause their loss! Indeed, cells that have absorbed crystals are poisoned. In addition, they ignite and develop a molecular complex called an inflammasome. This molecular complex acts as a factory for producing help signal for the immune system. The immune system, when it arrives at the affected joint, will then start cleaning it. Unfortunately, it is a far from delicate process and the joint is permanently damaged: and that’s what gout is [24].
From the outside, it takes the form of a severe swelling of the joint, an increase in its temperature and a gradual loss of mobility. This is called acute arthritis, a very painful phenomenon[25]. These inflammations occur by seizures and are not permanent. Nevertheless, if the phenomenon persists, nodules, a kind of permanent but not painful swelling, may form, thus adding an additional handicap[26]. The most affected joint is the big toe, which most often prevents patients with gout from moving normally[27].
However, let’s not panic right away: gout is treatable. There are many treatments available to reduce uric acid levels in the blood and, in the worst case, uric acid crystals can be removed by surgery[24]. Thus, despite the obvious discomfort it represents, gout is one of the least dangerous age-related metabolic diseases.
Metabolism and aging: anemia, when the metabolism suffocates
It is probably not unknown to you that to live, we must breathe oxygen. Well, the reason is metabolic! Indeed, oxygen is essential to our mitochondria, which are present in each of our cells. They use it to make ATP, the fuel for the metabolism. Glucose is one of the main sources of energy used to produce ATP. If we compare ATP to the fuel our cells consume, glucose would be a form of crude oil and mitochondria the refinery. But here we are, without oxygen, the refinery of our cells breaks down.
Metabolism and aging: the challenge of delivering dioxygen
For living beings composed of only one or a few cells, getting oxygen is easy. It simply diffuses from the external environment into the cells. But for organisms like us, made up of billions of cells all needing oxygen, things get complicated. In our case, oxygen enters only through the lungs. But how do we get it all over our bodies? This is one of the main roles of our circulatory system, which makes blood travel through our body.
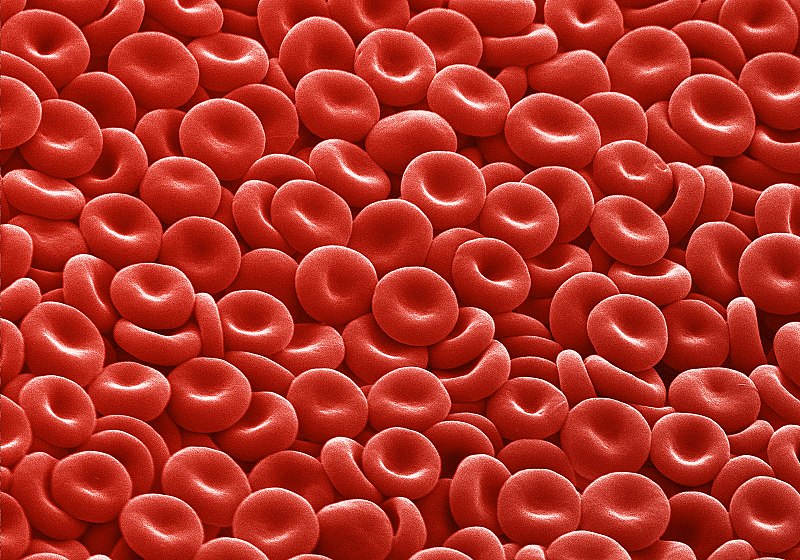
Oxygen is transported there by cells, commonly known as red blood cells. To carry oxygen, they use a protein, hemoglobin. This acts as a real container for oxygen. No hemoglobin, no oxygen transport, no cell survival. A detail that may seem insignificant but is of great importance: hemoglobin uses iron to fix oxygen, which gives the blood its red colour.
Unfortunately, as we age, the amount of hemoglobin in the blood can decrease: we call it anemia.
Metabolism and aging: anemia, aging and hemoglobin loss
Anemia affects up to 11% of people over 65[28] and more than 20% of people over 85[29]. But what are the causes? To reduce the amount of hemoglobin, there are two main ways: decrease the number of cells that carry it (red blood cells) or decrease the amount of hemoglobin present in the cells that carry it.
Metabolism and aging: red blood cells count can diminish with age
Red blood cells are renewed by blood stem cells: hematopoietic stem cells. Only, to do this, these stem cells must receive a hormone, EPO. This one is made by the kidneys. However, aging is sometimes accompanied by kidney failure, and therefore a loss of EPO production, ultimately leading to a decrease in the number of red blood cells[30]. Another reason for the decrease in red blood cells is also a depletion over time of the number of stem cells, a depletion from which hematopoietic stem cells are not exempt[31].
Metabolism and aging: no iron, no red blood cells!
As explained above, to fix oxygen and to function, hemoglobin needs iron. Unfortunately, aging is sometimes accompanied by gastrointestinal disorders involving a release of blood in the digestive tract. The iron in the blood is then lost. This is one of the main causes of iron-deficiency anemia in the elderly[32]. Moreover, chronic inflammation, which is more frequent in the elderly, is indirectly responsible for a lack of iron fixation by hemoglobin. Indeed, in the event of inflammation, the liver can produce a molecule, hepicidine. This prevents the intestine from absorbing iron and at the same time encourages its recirculation in the body, preventing hemoglobin from using it. This is called inflammatory anemia[33].
Thus, in two different ways, aging can cause a decrease in the amount of hemoglobin in the blood, and therefore problems in oxygen transport. But what are the consequences?
Metabolism and aging: consequences of anemia on the metabolism
As one might guess, anemia in the elderly has serious consequences. In patients over 85 years of age, it would multiply the mortality rate by 1.6 in women and by nearly 2.3 in men[34]. This is understandable when our metabolism is impacted, anemia is accompanied by significant issues, both functional[35], and cognitive[36], and increased risk of falls in the elderly[37].
In addition, anemia itself is sometimes difficult to treat. Indeed, the only solution is to solve the problem that caused it, which can sometimes be complicated. Thus, treating a patient with EPO when he has a hematopoietic stem cell deficiency would be ineffective. In another example, transfusing a patient with hemoglobin-laden blood would be unnecessary if he or she suffers from gastrointestinal bleeding[32].
Thus, metabolic diseases can take all kinds of forms. How can we suspect that joint pain such as gout can have its origins in the misuse of our body’s energy? However, recent advances in metabolic research are gradually allowing us to accurately identify the causes and mechanisms of these diseases. It is by studying them, by increasing the accuracy of our knowledge about them, that we will be able to overcome them in the future.
Diseases of aging: our articles
Baptiste Tesson

Author
Auteur
Baptiste is studying biology at the École Normale Supérieure de Lyon and bioengineering at the École Polytechnique Fédérale de Lausanne. He worked on the optimization of Cas9 as a tool for genome editing and on the emergence of blood stem cells in the zebrafish. He currently works on the patterning of the muscles, also in the zebrafish. He plans on doing a PhD in developmental biology.
More about the Long Long Life team
Baptiste étudie la biologie à l’École Normale Supérieure de Lyon et la bioingénierie à l’École Polytechnique Fédérale de Lausanne. Il a travaillé sur l’optimisation de la protéine Cas9 comme outil de modification de génomes et sur le développement des cellules souches du sang chez le poisson zèbre et travaille actuellement sur la mise en place des muscles chez le même animal. Il projette de réaliser un doctorat axé vers le développement animal.
En savoir plus sur l’équipe de Long Long Life
References:
[1] Virally, M., Laloi-Michelin, M., Kevorkian, J. P., Bitu, J., & Guillausseau, P. J. (2011). Specificities of type 2 diabetes in the elderly. Sang Thrombose Vaisseaux, 23(8), 409-415.
[2] Wilcox G. Insulin and Insulin Resistance. Clinical Biochemist Reviews. 2005;26(2):19-39.
[3] Muller, D. C., Elahi, D., Tobin, J. D. & Andres, R. Insulin response during the oral glucose tolerance test: the role of age, sex, body fat and the pattern of fat distribution. Aging (Milano) 8, 13–21 (1996).
[4] DeFronzo, R. A., Ferrannini, E., Groop, L., Henry, R. R., Herman, W. H., Holst, J. J., … & Simonson, D. C. (2015). Type 2 diabetes mellitus. Nature reviews Disease primers, 1, 15019.
[5] Katsarou, A., Gudbjörnsdottir, S., Rawshani, A., Dabelea, D., Bonifacio, E., Anderson, B. J., … & Lernmark, Å. (2017). Type 1 diabetes mellitus. Nature reviews Disease primers, 3, 17016.
[6] Marchetti, P. et al. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia 50, 2486–2494 (2007).
[7] Marchetti, P. & Masini, M. Autophagy and the pancreatic beta-cell in human type 2 diabetes. Autophagy 5, 1055–1056 (2009).
[8] Deng, S. et al. Structural and functional abnormalities in the islets isolated from type 2 diabetic subjects. Diabetes 53, 624–632 (2004)
[9] Copps, K. D. & White, M. F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 55, 2565–2582 (2012).
[10] Hiratani, K. et al. Roles of mTOR and JNK in serine phosphorylation, translocation, and degradation of IRS‑1. Biochem. Biophys. Res. Commun. 335, 836–842 (2005)
[11] Krüger, M. et al. Dissection of the insulin signaling pathway via quantitative phosphoproteomics. Proc. Natl Acad. Sci. USA 105, 2451–2456 (2008).
[12] Petersen, K. F. et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300, 1140–1142 (2003).
[13] Romeo, G. R., Lee, J. & Shoelson, S. E. Metabolic syndrome, insulin resistance, and roles of inflammation — mechanisms and therapeutic targets. Arterioscler. Thromb. Vasc. Biol. 32, 1771–1776 (2012).
[14] Herschkovitz, A. et al. Common inhibitory serine sites phosphorylated by IRS‑1 kinases, triggered by insulin and inducers of insulin resistance. J. Biol. Chem. 282, 18018–18027 (2007).
[15] https://infos-diabete.com/diabete/diabete-de-type-2/
[16] DeFronzo, R. A. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58, 773–795 (2009).
[17] Vasavada, R. C. et al. Protein kinase C‑ζ activation markedly enhances β-cell proliferation: an essential role in growth factor mediated β-cell mitogenesis. Diabetes 56, 2732–2743 (2007).
[18] Gaich, G. et al. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 18, 333–340 (2013).
[19] Angstadt, Carol N. (4 December 1997). “Purine and Pyrimidine Metabolism: Purine Catabolism”. NetBiochem.
[20] Perez-Ruiz F, Calabozo M, Erauskin GG, Ruibal A, Herrero-Beites AM. Renal underexcretion of uric acid is present in patients with apparent high urinary uric acid output. Arthritis Rheum 2002; 47: 610–13.
[21] Mandal AK, Mount DB. The molecular physiology of uric acid homeostasis. Annu Rev Physiol 2015; 77: 323–45.
[22] Kuo C-F, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol 2015; 11: 649–62.
[23] Loeb JN. The infl uence of temperature on the solubility of monosodium urate. Arthritis Rheum 1972; 15: 189–92.
[24] N. Dalbeth, T. R. Merriman, and L. K. Stamp, “Gout,” The Lancet, vol. 388, no. 10055, pp. 2039–2052, 2016.
[25] Taylor WJ, Fransen J, Jansen TL, et al. Study for Updated Gout Classification Criteria (SUGAR): identifi cation of features to classify gout. Arthritis Care Res (Hoboken) 2015; 67: 1304–15.
[26] Aati O, Taylor WJ, Siegert RJ, et al. Development of a patient-reported outcome measure of tophus burden: the tophus impact questionnaire (TIQ-20). Ann Rheum Dis 2015; 74: 2144–50.
[27] Grahame R, Scott JT. Clinical survey of 354 patients with gout. Ann Rheum Dis 1970; 29: 461–68
[28] Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States : Evidence for a high rate of unexplained anemia. Blood 2004;104:2263-8.
[29] Patel KV. Epidemiology of anemia in older adults. Semin Hematol 2008;45:210-7.
[30] McClellan W, Aronoff SL, Bolton WK, et al. The prevalence of anemia in patients with chronic kidney disease. Curr Med Res Opin 2004;20:1501-10.
[31] Geiger H, Van Zant G. The aging of lympho-hematopoietic stem cells. Nat Immunol 2002;3:329-33.
[32] Emilia Frangos, Kaveh Samii, Jean-Jacques Perrenoud, Ulrich M. Vischer. L’anémie du sujet âgé : une pathologie fréquente à ne pas banaliser. Rev Med Suisse 2010; volume 6.2125–2129.
[33] Lasocki S, Baron G, Driss F, et al. Diagnostic accuracy of serum hepcidin for iron deficiency in critically ill patients with anemia. Intensive Care Med 2010;36: 1044-8.
[34] Izaks GJ, Westendorp RG, Knook DL. The definition of anemia in older persons. JAMA 1999;281:1714-7.
[35] Thein M, Ershler WB, Artz AS, et al. Diminished quality of life and physical function in community-dwelling elderly with anemia. Medicine (Baltimore) 2009;88: 107-14.
[36] Zamboni V, Cesari M, Zuccala G, et al. Anemia and cognitive performance in hospitalized older patients : Results from the GIFA study. Int J Geriatr Psychiatry 2006;21:529-34.
[37] Dharmarajan TS, Avula S, Norkus EP. Anemia increases risk for falls in hospitalized older adults : An evaluation of falls in 362 hospitalized, ambulatory, long-term care, and community patients. J Am Med Dir Assoc 2006;7:287-93.









